|
Evonik Launches Care Product Lines Designed Specifically For
Medical Market
Polymer’s Biocompatibility, Chemical Resistance Excellent For Medical Devices
Evonik Corporation launched VESTAMID® Care, TROGAMID® Care and VESTAKEEP® Care
product lines of high-performance polymers, which are designed specifically for
use in medical devices.
The properties of VESTAMID® Care, TROGAMID® Care and VESTAKEEP® Care polymers
include high elasticity, impact and chemical resistance, and durability, making
them excellent materials for medical devices including catheters, surgical
instruments and hearing aids.
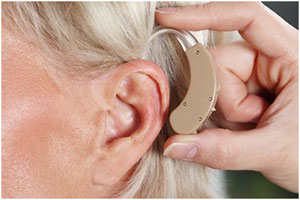
TROGAMID® Care is an excellent material
for hearing aids due to its high chemical resistance.
“Evonik works closely with its customers to develop materials which meet
application requirements and market needs,” said Jeff Smith, vice president and
general manager of Evonik’s High Performance Polymers business line. “Evonik is
one of the leaders in polymer design technology and assists its customers
throughout the product development process. We created these product lines to
help our customers’ meet their medical device manufacturing needs.”
Care products are biocompatible and are customized depending on the technical
requirements of the medical device based on characteristics such as either
flexible or rigid, and either transparent or translucent.
http://north-america.evonik.com/region/north_america/en/
media/newsreleases/pages/news-details.aspx?newsid=41167
New Silicone Film From Wacker For Wound Dressing
Wacker, the Germany-based chemical group, launched a silicone film for medical
applications at Compamed 2013. Silpuran Film can be used as a flexible wound
dressing, as a breathable membrane or as a dielectric elastomer in sensors and
artificial muscles.
Also launched at the show was Silpuran Aux 8250 RO, a silicone masterbatch
containing a barium sulphate contrast medium for formulating silicone compounds
for radio-opaque tubes and catheters.
Silpuran Film consists of an ultrapure silicone rubber and is produced in
thicknesses down to 20 microns. The films are manufactured under cleanroom
conditions to avoid the risk of contamination. It is free of organic
plasticisers and stabilisers and has passed selected tests for biocompatibility
according to ISO 10993 and US Pharmacopeia Class VI. It is chemically inert,
heat-resistant, flexible at low temperatures, elastic, tear-resistant and
permeable to gas but not to water.
Silpuran Aux 8250 RO has a barium sulphate content of 75%. Compounding
incorporates the masterbatch into the silicone material directly, without
additional processing. The company claims that because the contrast medium comes
predispersed in silicone, Silpuran Aux 8250 RO mixes much more easily.
http://www.europeanplasticsnews.com/subscriber/
newscat2.html?cat=1&channel=310&id=3984
|
